

Unplugging the refrigerator would lower the heat in the kitchen the most,definitely more than keeping the door open. In fact, it is doing the opposite: the harder the refrigerator has to work, due to the open door, the more heat it is dumping into the room.
Short of buying an air conditioner, what could they do with the refrigerator to reduce the heat in the kitchen?8This is most certainly costing them money without cooling the room. Will this at least achieve the womens desired result (keeping cool) regardless of how much it costs? Is this a good idea or a bad idea, as far as their electric bill is concerned? The women is standing in front of the open door of the refrigerator, in an obvious attempt to cool off. W = 200 JQC = 300 JQH = W + QC= 200 J + 300 J= 500 JĦRefrigerators and Heat PumpsA refrigerator is also a form of a heat pump.It also moves heat from a cooler reservoir to a warmer reservoir by means of work supplied from some external source.It keeps food cold by pumping heat out of the cooler interior of the refrigerator into the warmer room.An electric motor or gas-powered engine does the necessary work.ħA television commercial shows a couple in the middle of a heat wave, discussing buying an air conditioner.


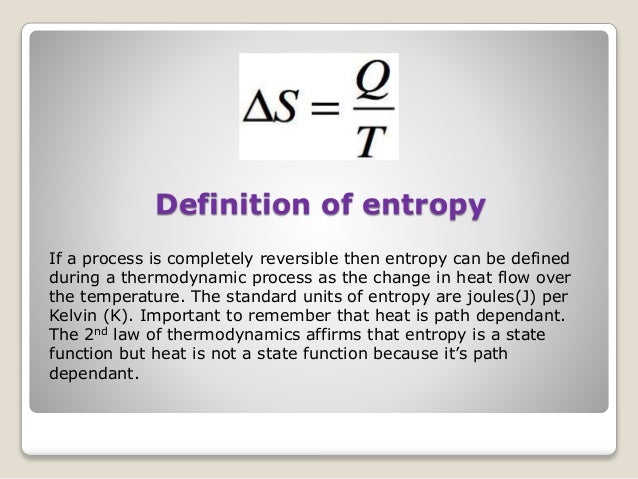
How much heat would be delivered to the higher-temperature reservoir?100 J200 J300 J500 J Therefore, no engine can have a greater efficiency than a Carnot engine operating between the same two temperatures.1Second Law of ThermodynamicsAn engine with an efficiency greater than the Carnot engine would produce a greater amount of work than the Carnot engine, for the same amount of heat input QH.Some of this work could be used to run the Carnot engine in reverse, returning the heat released by the first engine to the higher-temperature reservoir.ĢSecond Law of ThermodynamicsThe remaining work Wexcess would be available for external use, and no heat would end up in the lower-temperature reservoir.The two engines would take a small quantity of heat from the higher-temperature reservoir and convert it completely to work.This would violate the second law of thermodynamics.ģRefrigerators, Heat Pumps, and EntropyIf a heat engine is run in reverse, then work W is done on the engine as heat QC is removed from the lower-temperature reservoir and a greater quantity of heat QH is released to the higher-temperature reservoir.A device that moves heat from a cooler reservoir to a warmer reservoir by means of work supplied from some external source is called a heat pump.ĤThe first law of thermodynamics requires that, for a complete cycle, the heat released at the higher temperature must equal the energy put into the engine in the form of both heat and work.The engine returns to its initial condition at the end of each cycle the internal energy of the engine does not change.More heat is released at the higher temperature than was taken in at the lower temperature.ĥA heat pump uses 200 J of work to remove 300 J of heat from the lower-temperature reservoir. Second Law of ThermodynamicsNo engine, working in a continuous cycle, can take heat from a reservoir at a single temperature and convert that heat completely to work.


 0 kommentar(er)
0 kommentar(er)
